Due to the recent emergence of Acinetobacter as a platelet contaminant, the FDA has issued a warning to the healthcare community about sepsis from the transfusion of platelets contaminated with this bacteria. When the original PGD Test was developed, this species had not been reported as a platelet contaminant and as a result the PGD Test was not optimized for its detection. But with its emergence as a serious platelet contaminant that has been implicated in transfusion fatalities attributed to contaminated pathogen reduced platelets, Verax has implemented a product update to the Platelet PGDprime test. This update:
PGDprime preserves the performance of our proven Platelet PGD Test, while taking ease of use to a whole new level.
And Platelet PGDprime still delivers everything you like about the Platelet PGD Test.
* Vortex mixing is only needed with WBD post storage pools and single WBDs tested within four hours of transfusion.
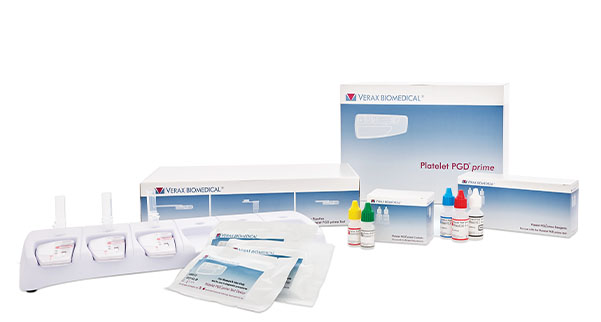
Platelet PGDprime includes everything you need to test your platelet inventory.
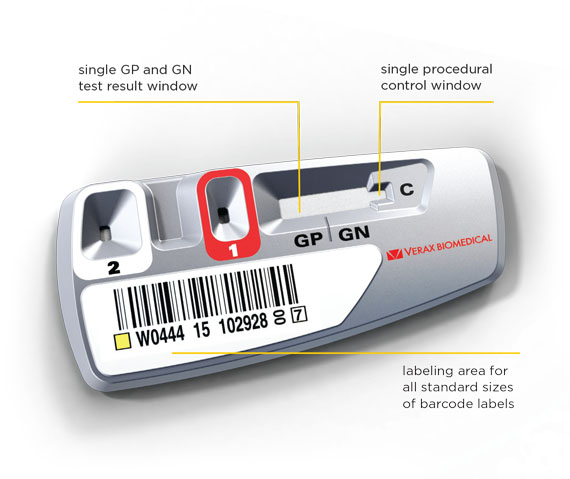
**Abbreviations for platelets tested: LR WBD = Leukoreduced Whole Blood Derived, LR WBDP = Leukoreduced Whole Blood Derived Pools, LRAP = Leukoreduced Apheresis Platelets, NLR WBD = Non-Leukoreduced Whole Blood Derived, NLR WDBP = Non-Leukoreduced Whole Blood Derived Pools, PAS = LRAPs suspended in PAS-C and plasma, PSP = Pre-Storage LR WBD Pools
***Reported both with (N = 503) and without (N = 502) the sample classified as Ind (indeterminate) because the Initially Reactive result for that sample was only followed up by a single additional test making it impossible to interpret as either Non-Reactive or Repeat Reactive. (Note: The single repeat test performed was Non-Reactive.)