The Company’s first product is the Platelet PGD Test for the detection of bacterial contamination in platelets. It is the only rapid test cleared by the FDA as a Safety Measure for leukoreduced apheresis platelets in plasma, or platelet additive solution as well as prestorage pooled platelets within 24 hours prior to transfusion. Other tests for the detection of bacteria are typically applied early in platelet unit life or ride their results for several days and have been demonstrated to offer detection rates of just 15-40%1,2. Use of the PGD test within 24 hours of transfusion offers the opportunity to interdict and prevent the transfusion of a proportion of severely contaminated units that pose a serious risk to patient safety and which are not reliably detected by existing culture tests for apheresis platelets. The test is also FDA cleared for single units of leukoreduced and non-leukoreduced whole blood derived platelets as well as pools of up to 6 of those units within 4 hours of transfusion.
The original Platelet PGD Test demonstrated an excellent observed specificity of 99.5% when testing leukoreduced apheresis platelets in a large multi-center study, but there is always room for improvement. Analysis of rare false positive PGD Test samples provided by customers gave us insight into their cause. That new insight has now translated into an updated Platelet PGD Test in which we have virtually eliminated this source of false reactivity — without degrading test performance in any way.
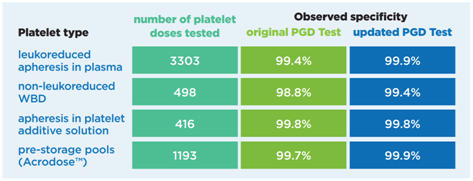
Results of external and internal validation studies of the updated Platelet PGD Test3:
This improved performance does not sacrifice the benefits the PGD Test already provides. You will continue to benefit from the advantages that make the PGD Test the preferred method for testing platelets — detection, ease of use, and 7-day dating — along with even greater specificity.
Watch DemoOver a 2 year period 18 US hospitals participated in a study using the PGD test on the day of transfusion. The objective of the study was to evaluate the test’s ability to detect bacterially contaminated units in the US apheresis inventory that tested negative for contamination by existing growth based QC tests. A total of 9 contaminated units were detected by PGD and confirmed as bacterially contaminated in a population of 27,620 leukoreduced apheresis units (1:3,069 doses tested). Seven of these 9 units were determined to have very high levels of contamination that have been associated with serious to fatal transfusion reactions4. All 9 units had previously tested negative by growth based QC methods applied earlier in unit life in conformance with all applicable AABB and CAP standards for bacterial testing. The study clearly demonstrated the ability of the Platelet PGD Test to detect and interdict contaminated units missed by current QC testing methods. The study findings have been published in the journal Transfusion.
Download the article here

The test consists of a disposable plastic cartridge that can be stored at room temperature and three dropper bottle sample pretreatment reagents. The Platelet PGD test procedure involves pre-treating a freshly collected 500uL platelet sample and applying it to the sample well on the test cartridge. Within approximately 20 minutes of sample addition, a pink colored line will appear in one of the two reading windows on the test cartridge if either Gram-positive or Gram-negative bacteria are detected in the sample above the cut-off level of the assay. Non-reactive samples show no color line in the result windows. Procedural Controls which change from yellow to blue violet when the test is ready to be interpreted are included at each end of the test cartridge. These allow visual confirmation that the appropriate volume of sample was added to the cartridge and that the test has run to completion.
1 Blajchman MA, Beckers EA. Dickmeiss E et al. Bacterial detection of platelets: current problems and possible resolutions. Transfus Med Rev 2005; 19(4):259-72
2 Pearce S, Rowe GP, Field SP, Screening of platelets for bacterial contamination at the Welsh Blood Service. Transfus Med 2011; 21(1):25-32
3 Platalet PGD Test package insert, Revision J
4 Jacobs MR et al. Relationship between bacterial load, species, virulence, and transfusion reactions with transfusion of bacterially contaminated platelets. Clin Infect Dis 2008; 45(8):1214-20

Distributed exclusively by Fisher Healthcare. To order or learn more click below.